Understanding the Sausage Conundrum: A Journey Through Carbon Chemistry
Written on
Chapter 1: The Challenge of Carbon Chemistry
Many of us have a love-hate relationship with the term "chemistry." It often conjures memories of complex lectures and formulas that faded from our minds after exams. However, the aim of this series is to shift that perspective, making chemistry accessible and engaging for everyone.
Previously, we examined the chaotic state of chemical bonding theories for organic molecules during the 19th century. The debates among various factions were intense and filled with sarcasm. To summarize the dilemma: outdated theories from binary inorganic compounds were misapplied to organic molecules. A contemporary chemist would quickly recognize the flaw; carbon, the cornerstone of organic chemistry, forms covalent bonds by sharing electrons, while inorganic compounds create ionic bonds through electron transfer. Attempting to apply inorganic bonding theories to organic compounds would inevitably result in confusion and failure.
If that explanation felt complicated, don't fret—what you need to grasp is that theorists were trying to impose unsuitable logic on organic molecules. Yet, it's important to appreciate that scientists were working with the limited knowledge of their time, primarily focused on inorganic compounds since the early 1700s. This struggle ultimately led to a pivotal realization regarding molecular structure, which was largely unknown and often overlooked. Understanding structure is crucial in organic chemistry, and this revelation was championed by August Kekulé.
Kekulé: A Name to Remember
The early years of Friedrich August Kekulé (1829–1896) are not widely documented, but he grew up in a middle-class household. Initially a student of architecture at Giessen (a name you might recognize), he shifted his focus to chemistry under the mentorship of the renowned Justus von Liebig. While Kekulé made significant contributions to chemistry, most of his work was theoretical, relying on existing data rather than conducting his own experiments, which some might consider a bit lazy.
In addition to his impressive name, Kekulé studied alongside many prominent chemists of the era, such as Dumas in France and Williamson in England. This exposure likely shaped his understanding of carbon's tetravalency—the concept that carbon can bond with four other atoms, including itself. This understanding was a logical evolution from previous theories and marked a crucial step towards grasping chemical structure and bonding. Until then, organic chemists struggled to reconcile "radical" theory with experimental data, yielding little success.
It's worth noting that the term "bonding" was not yet in common use, which is why the phrase "chemical combination" was prevalent. The true nature of chemical bonds—the physical connections between atoms—was still a mystery.
Kekulé's first representation of chemical combinations involved a linear arrangement of Dalton's circles, with carbon depicted as four connected circles, symbolizing its ability to form four bonds. In a playful twist, these representations resembled sausages.
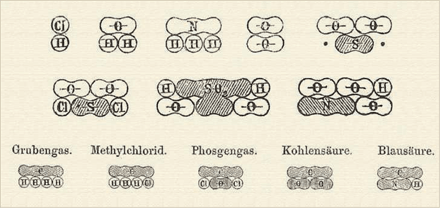
The Legacy of Kekulé's Sausages
Kekulé's original "sausages" are elusive, found only in his initial textbook—though I suspect he may have abandoned them due to the inevitable teasing. The illustration showcases how tetravalent carbon aligns with empirical data, displaying methane, chloromethane, phosgene, carbon dioxide, and hydrogen cyanide from left to right. Remarkably, these drawings accurately represent carbon's bonding in the mid-1800s.
However, the whimsical name "sausages" wouldn't suffice for a systematic approach to drawing organic molecules. A more refined method, introduced by Archibald Couper (1831–1892), initially employed dotted lines and later solid lines to signify bonds between carbon and other atoms. This framework was further refined by Alexander Crum Brown (1838–1922), leading to structures comprehensible to modern chemists.
Kekulé's work achieved two significant milestones: first, it unified various conflicting theories in organic chemistry, and second, it highlighted the critical importance of molecular structure in understanding compounds, especially organic ones. His research also clarified the concept of isomer formation and contributed to the modern definition of organic chemistry as "the study of carbon-containing compounds." While the complete understanding of how carbon bonds remains a work in progress, chemists were finally on the path to clarity.
Now, if only the issue of atomic weights could be resolved—an ongoing challenge for the past 50 years!
The first video, "Can We Reduce Our Reliance On Carbon? | The Carbon Conundrum | Full Episode," dives deep into the complexities surrounding carbon usage in modern society.
The second video, "Mastering Moisture Control: Building a Brain for Sausage Manufacturing," explores innovative techniques in the food production industry.